By Ken Butts
This is the article version of our webinar: Evaluating Fluorescent White Materials. It’s part of our on-demand series of Digital Color Management Fundamentals.
Have you ever wondered why your white clothing seems to glow under a blacklight, or why some white materials just seem to look whiter than others? What you’re experiencing is the effect of fluorescent whites.
What is a Fluorescent White Material?
A fluorescent white material is one that has been treated with special chemicals known as optical brightening agents (OBA), or sometimes referred to as fluorescent whitening agents (FWA). Because an OBA can be applied to colored materials as well, it is the more generic term of the two.
The average consumer is looking for white apparel that has a crisp, clean appearance, and this can be difficult to achieve in some materials, especially cotton. Even after cotton is bleached, it can still have a dull yellow appearance that makes it look dirty. The best way to make bleached cotton “whiter” is to “hide” the dull yellow appearance. A little color science can help with understanding how this is accomplished with OBAs. White materials appear yellow or dingy because they absorb blue energy from the light source and reflect yellow energy. To counter this effect, we could “add” more blue energy, and the result should be that the material looks whiter, i.e., cleaner and brighter, than it really is. The OBA does just that – it “adds” blue energy by converting invisible ultraviolet (UV) energy we can’t see into visible blue energy that we can see.
Evaluating Fluorescent Whites in a Light Cabinet
A key point to remember when evaluating a white sample treated with an OBA is that as the amount of UV energy present in the light source changes, the final appearance of the sample will change. This means we must be very careful in selecting the light source that will be used to evaluate the white sample. Normal daylight from the sun or from a daylight source in a light booth is rich in UV energy — much more than a fluorescent light source for example. If we are evaluating a fluorescent white in a light booth and use an incandescent or fluorescent light source, and then look at the sample in a daylight source, we should find that the sample appears much brighter and whiter in the daylight source.
The daylight source in the light booth may not, however, contain the same amount of UV energy that is found in normal sunlight, or from one light booth to another, in which case our visual evaluation will be inconsistent. It’s important to speak with your light cabinet manufacturer and find out if the UV content of the daylight source is controlled and corresponds to normal daylight. Evaluating fluorescent whites is going to be most effective when you are working with a light booth that has a daylight light source that approximates the amount of UV energy present in normal daylight.
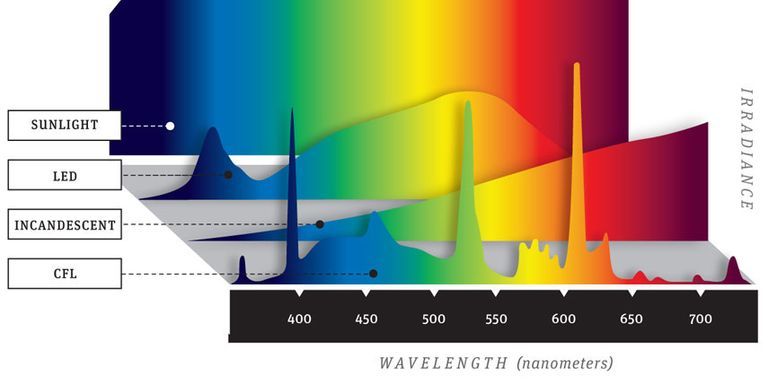
[Image Source: Popular Mechanics]
How to Make Effective Measurements of Fluorescent Whites
Because of the challenges in making consistent visual evaluations of white materials, it is often more effective to evaluate whiteness with a spectrophotometer. A spectrophotometer is used to measure how much visible light energy reflects from a material, and then reports this as percent reflectance (%R) in the wavelength range of 400-700 nanometers (nm). OBAs absorb ultraviolet (UV) energy in the region of the electromagnetic spectrum below 400 nanometers and reemit that energy in the visible portion of the spectrum. If you were to measure a fluorescent white on a spectrophotometer, you would see that the reflectance in the shorter wavelength blue region is greater than 100% – this is the “extra” blue energy that “hides” the yellow appearance of the material. As with the visual evaluation however, the whitening effect can vary depending upon how much UV energy is present in the light source of the spectrophotometer.
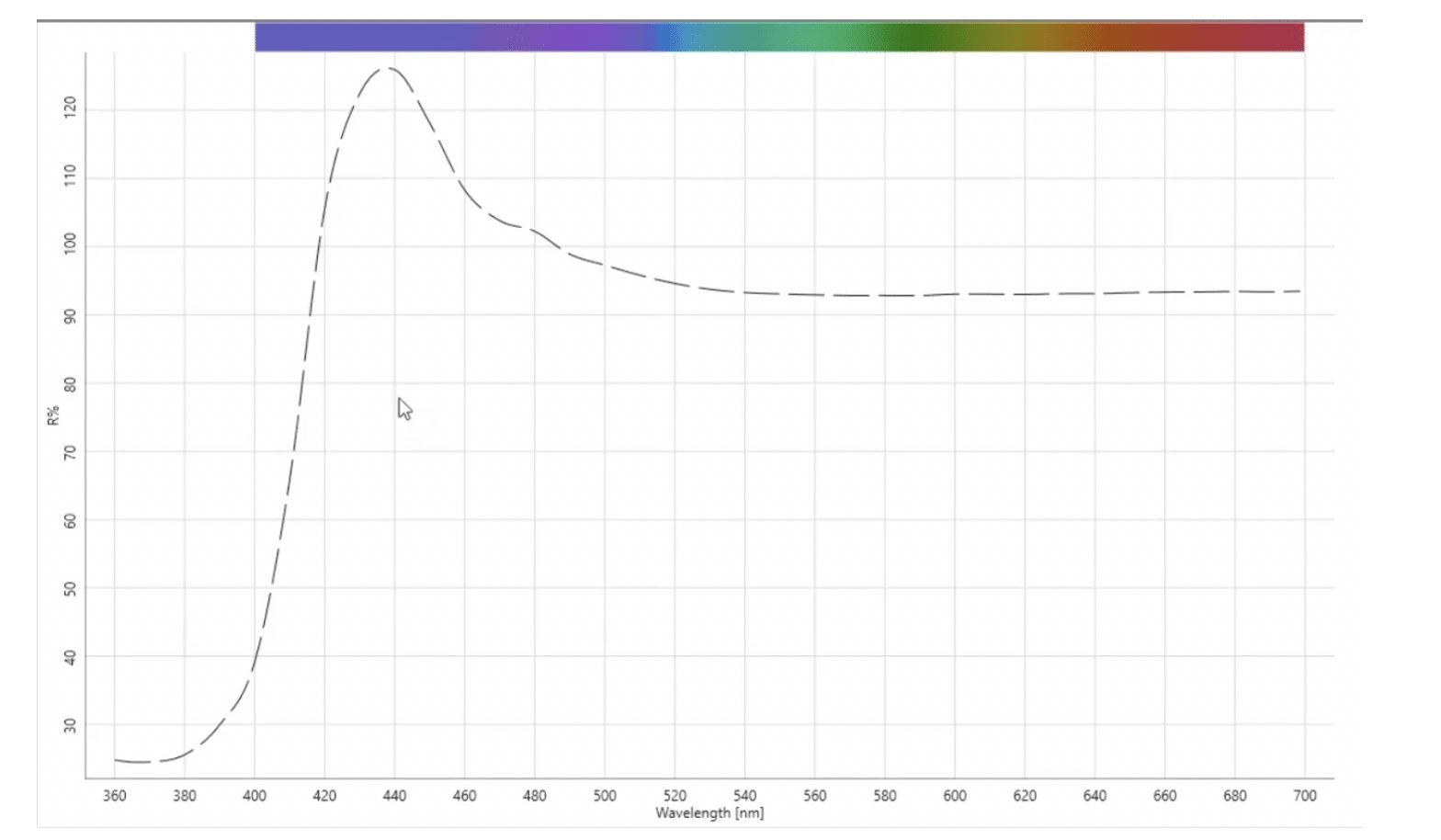
To effectively measure a fluorescent white sample, we must control the amount of UV energy that is present in the light source. For non-fluorescent materials we follow the
standard calibration process that uses a black trap and a white tile. These two calibration tools are designed to calibrate the visible portion of the spectrum only, and they do not help us control the amount of UV energy present in the flash lamp of the instrument.
Most high-end desktop spectrophotometers utilize what is called a xenon flash bulb. A xenon flash bulb, which is much like a camera flash bulb, is very rich in UV energy. In fact, it has a lot more UV energy than is present in normal daylight. So, if we were to measure a fluorescent white sample using 100% of that UV energy, we are going to get much more blue energy from the sample than we would see in our visual experience. And the numerical data would indicate that the sample is much whiter than it really is.
Calibrating your Spectrophotometer for Measuring Fluorescent Whites
So how do we calibrate the UV content of the spectrophotometer? One of the most commonly used tools for UV calibration is the AATCC textile UV calibration standard (TUVCS). This reference fabric is a square of several layers of fabric that has been treated with an FWA and then measured on a traceable instrument at AATCC so that we know exactly what the whiteness of this sample is supposed to be. The TUVCS is then used to calibrate the UV content of a spectrophotometer according to AATCC Evaluation Procedure 11 – Spectrophotometer UV Energy Calibration Procedure for Optically Brightened Textiles.
This method, along with guidance from your spectrophotometer manufacturer, will ensure that the UV content of your spectrophotometer is properly calibrated. It’s important to note that when calibrating UV with the TUVCS on a sphere spectrophotometer, the specular setting must be set to “included” (SCI) and the large area view (LAV) aperture should be used. We can then be confident that the results we produce are traceable back to international standards and consistent with the measurements made on other UV-calibrated spectrophotometers. Once you’ve calibrated the UV filter and you’re ready to start using Tools software, I recommend watching the webinar version of this article for a step-by-step, detailed tutorial (beginning at around 12 minutes and 20 seconds). We’ll start by measuring the standard, then move on to actually measuring samples and comparing results.
A special note in using the TUVCS: It does have an issue date and an expiration date. You do not want to use a standard that has expired because you will get inaccurate results. You’ll also want to be sure to handle the standard by the edges so as not to introduce any dirt or discoloration to the surface, and always store that standard in the BHT-free bag in which it came.
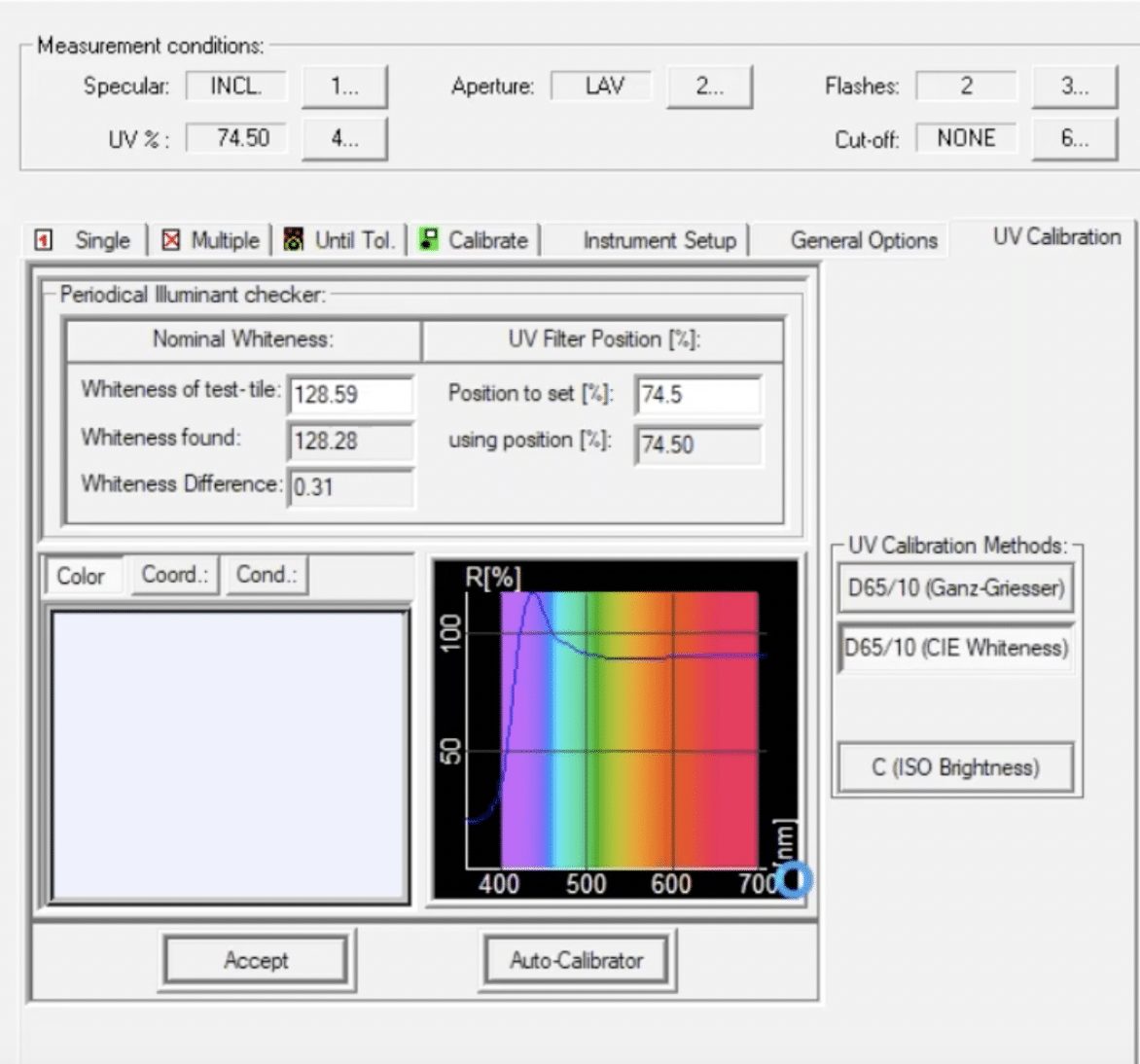
What is a Whiteness Index?
On its own, the reflectance data for a fluorescent white sample doesn’t give us the information we need to know how white a sample is or to make a decision about the acceptability of the sample. A specific calculation can be used to generate a “whiteness index” (WI) for a sample, which is a number that represents how white that sample is. There are several whiteness indices that have been developed over the years, with two of the most common being the CIE Whiteness Index and the Ganz-Griesser Whiteness Index. Typically, the bluer a sample is, the higher the whiteness index, and the more yellow it is, the lower the whiteness index.
The WI value for samples will vary, of course, depending upon the amount of OBA present and the starting purity of the sample, with higher values representing “whiter” samples. In addition to WI, these formulas usually offer a measure of the tint of the white, that is, whether the sample is reddish or greenish in cast.
Accounting for the Age of your Spectrophotometer
With a brand-new spectrophotometer from Datacolor, my experience is that the typical UV filter range requirement is between 60% and 80%. As the instrument ages, it will be necessary for the filter to open more to use more of the available UV energy in the bulb. Once the UV filter position reaches 90%, which means we’re using about 90% of the available UV energy, it’s time for you to contact Datacolor to discuss scheduling a service call to evaluate the instrument. At some point in the future, the filter may be allowing 100% of the UV energy, but you’re not going to be able to achieve the desired whiteness of the test standard.
More to note on instrument age: With most supply chains, you will find instruments of varying ages and we have seen that as an instrument ages, and depending upon the environmental conditions, the sphere may begin to yellow. That may also be true when an instrument is operated in a production environment with airborne contaminants. For fluorescent materials, we do find that the discoloration can affect the consistency of measured reflectance. We can compensate for some degree of that yellowing with our normal calibration process.
If you’re in a situation where the tint value of your measurement differs dramatically from the tint value of someone else’s measurement, then we should evaluate the ages of the spheres. It may be necessary to have one or both spheres replaced, or have them recoated in order to get better agreement between the two. As long as everyone is using instruments with spheres that are relatively new or have been replaced or recoated, then they will produce consistent results for tint value.
Working with Suppliers on Fluorescent Whites
The next step is to identify the acceptability ranges of whiteness and tint values for your samples. Once you’ve established numerical acceptability tolerances for whiteness index and you’ve asked your supplier to use a UV-calibrated instrument to measure their samples, they don’t actually have to evaluate the whiteness of those samples in the light booth. As long as they measure the samples accurately using the UV-calibrated instrument and they generate pass-fail results using your tolerances, then they can confidently either submit or rework those samples.
What if the Sample Still Looks too Dull or Dark?
In my experience over the past 20 years, some customers have set up their pass-fail system based on whiteness index and tint and still found that some samples didn’t look good visually even though they were within their numerical tolerances. In those cases, it can be helpful to consider use of an additional color difference element for pass-fail.
I’ve found some customers have had success using the CMC DL value, which is a measure of the difference in lightness and darkness between two samples. The whiteness index is based upon the area between approximately 420 and 460 nanometers, while the tint is based upon the increases and decreases or the high and the low areas in the rest of the reflectance curve. What we may find is that the curve of a sample has a very similar shape to the curve of standard, but it’s noticeably lower. That means that overall while it may have an acceptable whiteness index, the sample is going to look darker.
It is important to note that we do not recommend the use of DEcmc for pass-fail for fluorescent whites as it is not designed to accurately capture the influence of the FWA.
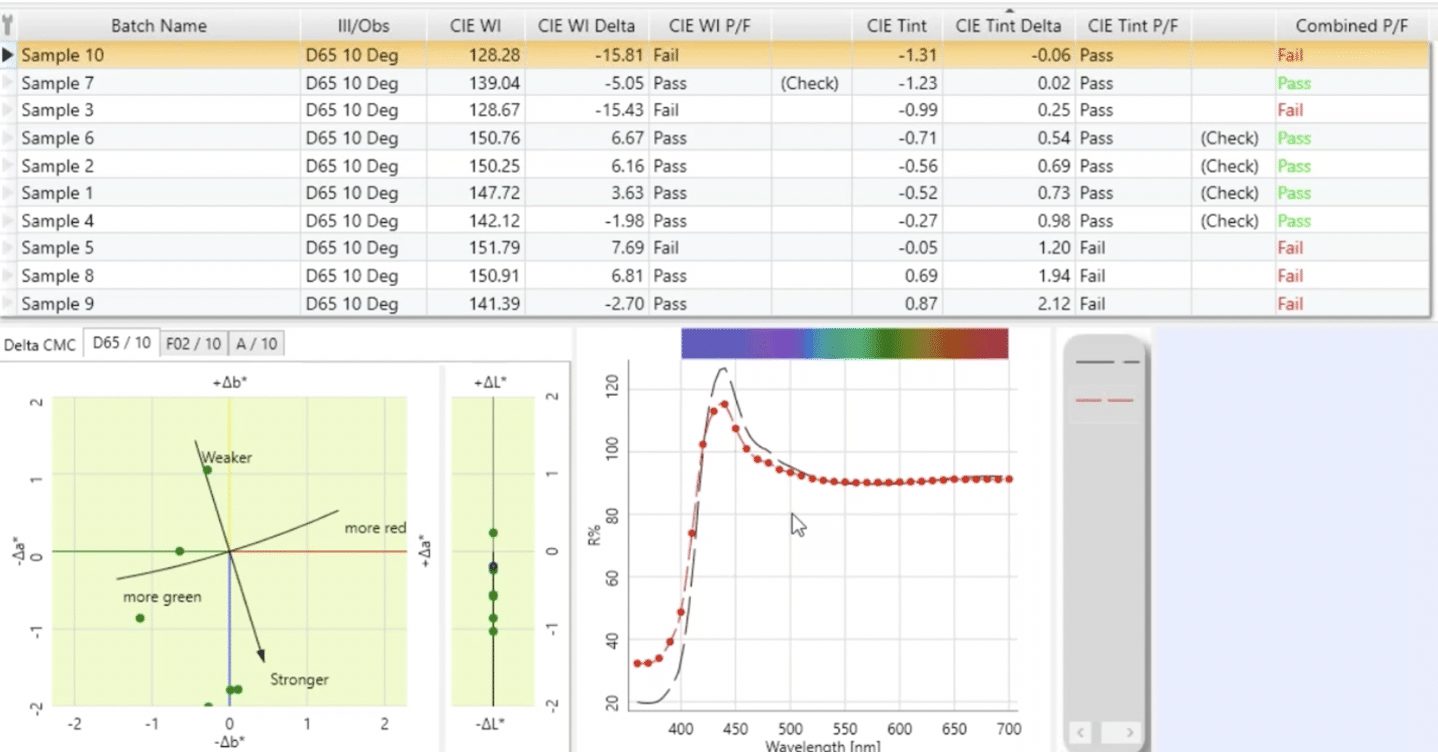
Can you See Fluorescent Whites on a Monitor?
Unfortunately, monitors can’t display fluorescent colors accurately. You might be able to pick up on some slight differences in the tint value or the depth of shade, but it’s just not going to be as bright as the actual sample, and in many cases the white will actually look blue on screen. So just a caution with any attempt to use the color on-screen component for evaluating fluorescent white.
Getting Repeatable Results for Optically Brightened White Materials
As we’ve seen, this is a challenge – especially when you’re making a visual assessment. But if you have calibrated your spectrophotometer using available UV calibration standards such as the TUVCS from AATCC, then you will get repeatable measurement results. Only then can you successfully establish a numerical pass-fail program for fluorescent whites.
If you have any questions about that process, contact our team of experts here or to me directly (find me on LinkedIn here).









